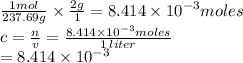Chemistry, 23.07.2019 06:00 helpmeplzandty
If you dissolve 2.00 g of nicl2*6h20 into a beaker of water and the final volume is 1.00 liters what will be the molar concentration of the solution?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2


Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
If you dissolve 2.00 g of nicl2*6h20 into a beaker of water and the final volume is 1.00 liters what...
Questions

Mathematics, 14.10.2019 17:30

Health, 14.10.2019 17:30