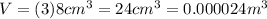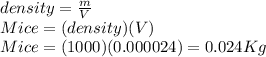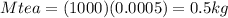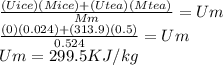Physics, 14.10.2019 17:30 guadalupemarlene2001
Suppose you are making yourself 0.50 liters of tea but its temperature is 75°c, which is too hot for you to drink. in order to cool its temperature, you immerse three ice cubes of the same dimension as the one in part b) in your tea. if the energy used to melt the ice came solely from the internal energy (i. e. temperature) of the tea, what would the reduced temperature of your tea be (where you may assume tea is made up entirely of water)? for simplicity, in this class you may generally use a value of 1000 kg/m3 for the density of water (although density, like many of the properties of water used in this problem have a relatively small temperature dependence).

Answers: 1


Another question on Physics

Physics, 22.06.2019 09:00
The pressure proportional to the area a- inversely b- directly c- increase d-decrease
Answers: 2

Physics, 22.06.2019 12:30
What would be the strength of earth's gravitational field at a point where an 80.0 kg astronaut would experience a 80% reduction in weight
Answers: 3

Physics, 22.06.2019 16:00
In which of the following is positive work done by a person on a suitcase
Answers: 1

Physics, 22.06.2019 18:30
Which form of cell division creates the sperm and egg, resulting in half of the chromosomes as the other cells?
Answers: 1
You know the right answer?
Suppose you are making yourself 0.50 liters of tea but its temperature is 75°c, which is too hot for...
Questions


Business, 05.10.2019 13:20

Mathematics, 05.10.2019 13:20

Mathematics, 05.10.2019 13:20





Mathematics, 05.10.2019 13:20

Mathematics, 05.10.2019 13:20

Biology, 05.10.2019 13:20


Biology, 05.10.2019 13:20






History, 05.10.2019 13:20

Social Studies, 05.10.2019 13:20