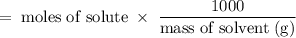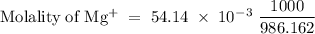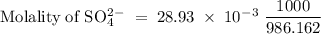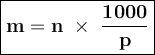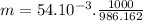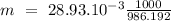Chemistry, 03.08.2019 04:00 morgaaaan651
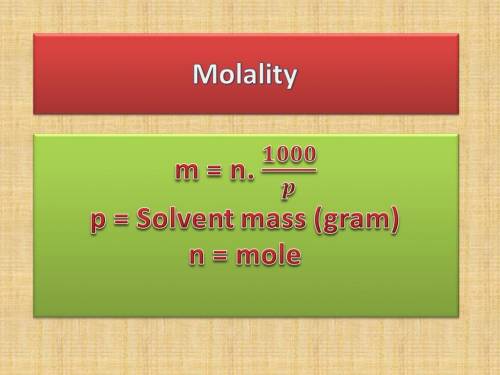
The following table lists molar concentrations of seven major ions in seawater. using a density of 1.022 g/ml for seawater, convert the concentrations for the two ions in the question below into molality. ions g/kg mm na+ 10.781 480.57 k+ 0.399 10.46 mg2+ 1.284 54.14 ca2+ 0.4119 10.53 cl- 19.353 559.40 so42- 2.712 28.93 hco3- 0.126 2.11 total 35.067 n/a magnesium ion = m? sulfate ion= m?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
The following table lists molar concentrations of seven major ions in seawater. using a density of 1...
Questions


Health, 16.11.2019 05:31

Biology, 16.11.2019 05:31

Mathematics, 16.11.2019 05:31













History, 16.11.2019 05:31



Physics, 16.11.2019 05:31

 volume
volume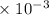 M.
M.