
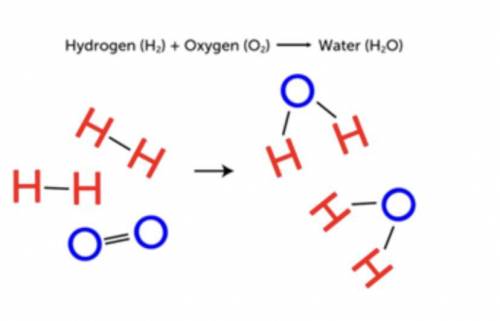
In Figure on the right, you see how hydrogen and oxygen combine chemically to form water.
a. How could you use chemical symbols and formulas to represent this reaction?
b. How many molecules of hydrogen and oxygen are involved in this reaction?
c. How many molecules of water are produced? How could you include these numbers in your representation of the reaction?
d. Which bonds were broken and which bonds were formed in this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
In Figure on the right, you see how hydrogen and oxygen combine chemically to form water.
a. How co...
Questions






















