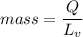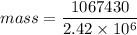Physics, 26.11.2019 20:31 adeliabujang8881
Aperson eats a container of strawberry yogurt. the nutritional facts label states that it contains 255 calories (1 calorie = 4186 j). what mass of perspiration would one have to lose to get rid of this energy? at body temperature, the latent heat of vaporization of water is 2.42 106 j/kg.

Answers: 3


Another question on Physics

Physics, 21.06.2019 17:20
Your 249 ml cup of coffee is too hot to drink when served at 82 degree celsius. what is the mass of an ice cube taken from a -21 degree celsius freezer, that will cool your coffee to a pleasant 54 degree celsius. specific heat of the water is 4190 j/kg c, specific heat of ice is 2090 j/kg c, heat of fusion of ice is 3.33 x 10^5 j/kg
Answers: 3

Physics, 21.06.2019 19:30
Molten iron fills a mould, which has a volume of 200 cm cubed. calculate the volume when the iron cools and solidifies. molten iron has a density of 7.0g/cm cubed. in its solid state, iron has a density of 8.0g/cm cubed.
Answers: 3

Physics, 22.06.2019 00:30
Occurs when an energy source transfers heat directly to another subject space; an example would be an object becoming warm by sitting in the sunshine
Answers: 1

Physics, 22.06.2019 22:30
Suppose that three astronomical objects, 1, 2, and 3 are observed to lie on a line, and the distance from object 1 to object 3 is d. object 1 has four times the mass of object 3, and seven times the mass of object 2. find the distance between objects 1 and 2 for which the net force on object 2 is zero.
Answers: 1
You know the right answer?
Aperson eats a container of strawberry yogurt. the nutritional facts label states that it contains 2...
Questions


Mathematics, 02.10.2019 09:30

Chemistry, 02.10.2019 09:30

Mathematics, 02.10.2019 09:30


English, 02.10.2019 09:30


Business, 02.10.2019 09:30

Mathematics, 02.10.2019 09:30


Mathematics, 02.10.2019 09:30

History, 02.10.2019 09:30

Biology, 02.10.2019 09:30

Mathematics, 02.10.2019 09:30

Computers and Technology, 02.10.2019 09:30

English, 02.10.2019 09:30

Mathematics, 02.10.2019 09:30

History, 02.10.2019 09:30

English, 02.10.2019 09:30