
Chemistry, 04.03.2021 06:40 samueldfhung
Reactants - Products Reactants Products
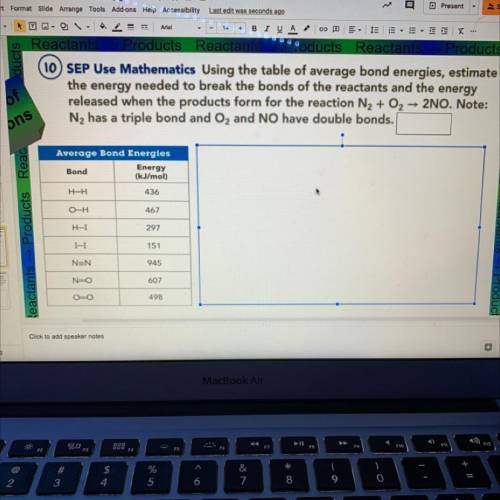
10 SEP Use Mathematics Using the table of average bond energies, estimate
the energy needed to break the bonds of the reactants and the energy
released when the products form for the reaction N2 + O2 2NO. Note:
N2 has a triple bond and O2 and NO have double bonds.
Reactants
of
ons
Average Bond Energies
Bond
Energy
(kJ/mol)
H-H
436
Products Reactant
O-H
467
H-I
297
I-I
151
eactants Products
NEN
945
N-O
607
→ Product
OO
498
Click to add speaker notes

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
Reactants - Products Reactants Products
10 SEP Use Mathematics Using the table of average bond ener...
Questions

Social Studies, 16.12.2019 15:31


Mathematics, 16.12.2019 15:31


Mathematics, 16.12.2019 15:31

Biology, 16.12.2019 15:31









Computers and Technology, 16.12.2019 15:31

Chemistry, 16.12.2019 15:31



Mathematics, 16.12.2019 15:31

English, 16.12.2019 15:31



