

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3
You know the right answer?
which two values for ∆g and e0cell correctly indicate a spontaneous reaction? a) ∆g = -89 kj, e0cel...
Questions

Physics, 20.04.2021 19:10


Mathematics, 20.04.2021 19:10

Mathematics, 20.04.2021 19:10

Mathematics, 20.04.2021 19:10


Computers and Technology, 20.04.2021 19:10

Computers and Technology, 20.04.2021 19:10

Mathematics, 20.04.2021 19:10

Mathematics, 20.04.2021 19:10

English, 20.04.2021 19:10

Spanish, 20.04.2021 19:10


History, 20.04.2021 19:10



Mathematics, 20.04.2021 19:10

Computers and Technology, 20.04.2021 19:10

Social Studies, 20.04.2021 19:10

Biology, 20.04.2021 19:10

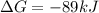 and
and 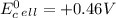
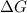 stands for Gibbs free energy equation. A reaction is spontaneous if
stands for Gibbs free energy equation. A reaction is spontaneous if 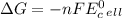
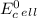 is standard cell potential.
is standard cell potential. 

