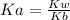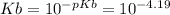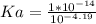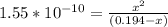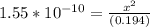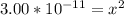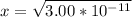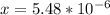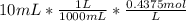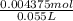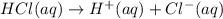The reaction between trimethylamine and HCl can be written as
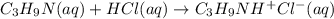
I) When 10 mL of HCl is added.
We have 25 mL of 0.3500 M trimethylamine solution. The moles can be calculated as,
Moles of trimethylamine = 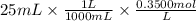
Moles of trimethylamine = 0.00875
Moles of HCl = 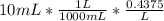
Moles of HCl = 0.004375
Let us set up an ICE table to find moles of C₃H₉NHCl at equilibrium.
Please refer to ICE table 1
From the ICE table we have
mol C₃H₉N = 0.004375
mol C₃H₉NHCl = 0.004375
This is a mixture of weak base C₃H₉N and its conjugate C₃H₉NHCl which represents a buffer.
The pH of the buffer can be calculated using Henderson-Hasselbalch equation which is given below.
![pH = pKa + log \frac{[Base]}{[Acid]}](/tpl/images/0420/7117/2cc82.png)
In our example acid is C₃H₉NHCl and base is C₃H₉N.
pKa can be calculated as
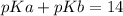
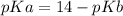
pKa = 14-4.19
pKa = 9.81
Let us plug in the values in Henderson equation
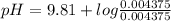
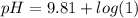
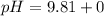
pH = 9.81
pH of the mixture when 10 mL of HCl is added is 9.81
II) 20 mL of HCl is added
Initial moles of C₃H₉N are 0.00875
Moles of HCl = 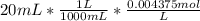
Moles of HCl = 0.00875
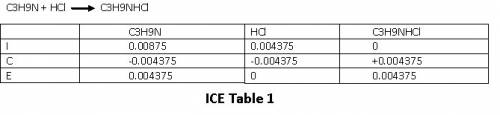
We can see that the moles of C₃H₉N and moles of HCl are equal, that means we have reached equivalence point.
At equivalence point, acid and base completely neutralize each other and the only species that is present is C₃H₉NHCl.
Please refer ICE table 2.
At equilibrium , we have 0.00875 mol C₃H₉NHCl.
The total volume at equilibrium is 25 mL of C₃H₉N + 20 mL HCl = 45 mL= 0.045 L
Concentration of C₃H₉NHCl at equilibrium is = 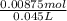
Concentration of C₃H₉NHCl = 0.194 M
C₃H₉NHCl is a strong salt and dissociates as follows.
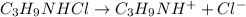
C₃H₉NH⁺ is a weak conjugate acid and has a tendency to react with water.
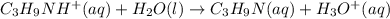
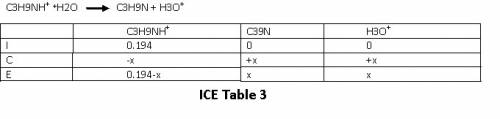
Let us set up an ICE table for this reaction .
Refer ICE table 3
From the ICE table we have equilibrium concentrations as
[C₃H₉NH⁺]eq = 0.194-x
[C₃H₉N]eq = x
[H₃O⁺] = x
The equilibrium constant equation can be set as
![Keq = \frac{[C_{3}H_{9}N] [H_{3}O^{+}]}{[C_{3}H_{9}NH^{+}]}](/tpl/images/0420/7117/a1869.png)
The above reaction is acid dissociation because C₃H₉NH⁺ is donating H⁺ ion.
Keq for the above reaction is Ka which can be calculated as follows.
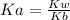
Kw is ionic constant of water which is 1 x 10⁻¹⁴.
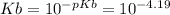
Let us plug in the values.
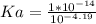
Ka = 1.55 x 10⁻¹⁰
Let us plug in these values in equilibrium constant equation.
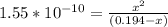
Since the equilibrium constant is very small, we will assume 0.194-x = 0.194.
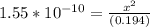
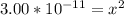
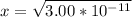
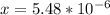
From the ICE table we know that x = [H₃O⁺]
So we have [H₃O⁺] = 5.48 x 10⁻⁶ M
pH = -log [H₃O⁺]
pH = -log ( 5.48 x 10⁻⁶)
pH = 5.26
pH when 20 mL of HCl is added is 5.26
III) 30 mL of HCl is added.
We know that the equilibrium volume of HCl is 20 mL.
That means 20 mL of HCl is needed to neutralize the given amount of trimethylamine.
Volume of HCl in excess = Volume added - Equilibrium volume
Volume of HCl in excess = 10 mL
Moles of HCl in 10 mL = 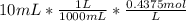
Moles of excess HCl = 0.004375
Total volume of reaction mixture = 25 mL trimethylamine + 30 mL HCl = 55 mL = 0.055 L
Concentration of HCl = 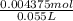
Concentration of HCl = 0.0795M
HCl is a strong acid and dissociates completely forming H⁺
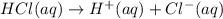
Mole ratio of HCl and H⁺ is 1:1
Therefore concentration of [H⁺] is 0.0795M
pH = - log [H⁺]
pH = -log ( 0.0795)
pH = 1.10
pH when 30 mL of HCl is added is 1.10




























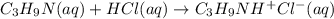
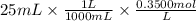
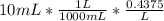
![pH = pKa + log \frac{[Base]}{[Acid]}](/tpl/images/0420/7117/2cc82.png)
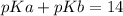
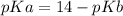
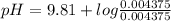
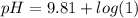
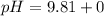
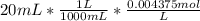
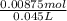
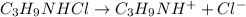
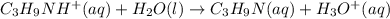
![Keq = \frac{[C_{3}H_{9}N] [H_{3}O^{+}]}{[C_{3}H_{9}NH^{+}]}](/tpl/images/0420/7117/a1869.png)