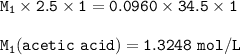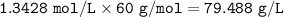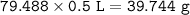Chemistry, 15.11.2020 21:10 Jonny13Diaz
If 2.50 ml of vinegar requires 34.50 ml of 0.0960 M NaOH to reach the equivalence point in a titration, how many grams of acetic acid are in 500.0 ml of this vinegar? Density of vinegar=1.01 g ml

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
If 2.50 ml of vinegar requires 34.50 ml of 0.0960 M NaOH to reach the equivalence point in a titrati...
Questions


Mathematics, 28.01.2020 20:13

Mathematics, 28.01.2020 20:13


Mathematics, 28.01.2020 20:13



Biology, 28.01.2020 20:13

Mathematics, 28.01.2020 20:13

Mathematics, 28.01.2020 20:13


Mathematics, 28.01.2020 20:13


Physics, 28.01.2020 20:13


Geography, 28.01.2020 20:13

Chemistry, 28.01.2020 20:13