
For the reactions system 2H2(g) + S2(g) 2H2S(g), a 1.00-liter vessel is found to contain 0.5 mole of H2 , 0.02 mole of S2, and 68.5 moles of H2S. What is the equilibrium constant expression? What are the chemical formulas, not numbers. (Enter subscripts after the letters: for example, H2O = Hs2O. Also, don't forget to use the proper chemical shorthand for chemical symbols. For instance, chlorine = Cl but not cl or cL.) Please and thank you!! :) NOT IN Numbers
This Image is how I have to answer it
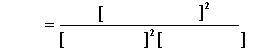

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
For the reactions system 2H2(g) + S2(g) 2H2S(g), a 1.00-liter vessel is found to contain 0.5 mole of...
Questions



Mathematics, 10.02.2020 23:31

Mathematics, 10.02.2020 23:31


Mathematics, 10.02.2020 23:31









Health, 10.02.2020 23:31

Mathematics, 10.02.2020 23:31


Physics, 10.02.2020 23:31


Mathematics, 10.02.2020 23:31




