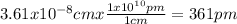Chemistry, 10.02.2020 23:31 jeffreystarks
Copper crystallizes in a face-centered cubic lattice (the Cu atoms are at the lattice points and at the face centers). If the density of the metal is 8.96 g/cm3, what is the unit cell edge length in pm? × 10 pm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Copper crystallizes in a face-centered cubic lattice (the Cu atoms are at the lattice points and at...
Questions














Mathematics, 17.08.2019 20:20




History, 17.08.2019 20:20


Law, 17.08.2019 20:20

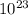 atoms/mol
atoms/mol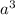 , which can be obtained through the relationship:
, which can be obtained through the relationship: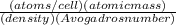
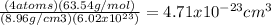
![\sqrt[3]{4.71x10^{-23}cm^{3} }=3.61x10^{-8}cm](/tpl/images/0505/4719/2ee6e.png)
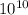 pm
pm