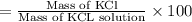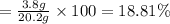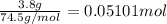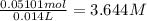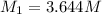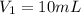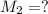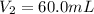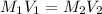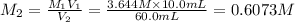Chemistry, 10.03.2020 03:19 jbrown76241
In a laboratory experiment, a 14.0 mL sample of KCl solution is poured into an evaporating dish with a mass of 24.10 g. The combined mass of the evaporating dish and KCl solution is 44.30 g. After heating, the evaporating dish and dry KCl have a combined mass of 27.90 g.
(a) What is the mass percent (m/m) of the KCl solution?
(b) What is the molarity ( M) of the KCl solution?
(c) If water is added to 10.0 mL of the initial KCl solution to give a final volume of 60.0 mL, what is the molarity of the diluted KCl solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

You know the right answer?
In a laboratory experiment, a 14.0 mL sample of KCl solution is poured into an evaporating dish with...
Questions







Computers and Technology, 30.08.2019 16:10

Engineering, 30.08.2019 16:10

Computers and Technology, 30.08.2019 16:10

Computers and Technology, 30.08.2019 16:10

Chemistry, 30.08.2019 16:10





Computers and Technology, 30.08.2019 16:10

Physics, 30.08.2019 16:10