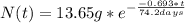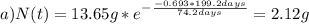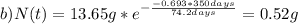Chemistry, 30.08.2019 16:10 hlgerardip4wbhx
The half-life for the radioactive decay of iridium-192 is 74.2 days. calculate the amount in grams of ir-192 that will be left from a 13.65g sample after a) 199.2 days b) 350 days

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1


Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
The half-life for the radioactive decay of iridium-192 is 74.2 days. calculate the amount in grams o...
Questions


Mathematics, 27.01.2020 04:31

Mathematics, 27.01.2020 04:31

Biology, 27.01.2020 04:31

Mathematics, 27.01.2020 04:31

Geography, 27.01.2020 04:31





Mathematics, 27.01.2020 04:31


Mathematics, 27.01.2020 04:31

Business, 27.01.2020 04:31


Mathematics, 27.01.2020 04:31


Mathematics, 27.01.2020 04:31

Medicine, 27.01.2020 04:31

Biology, 27.01.2020 04:31

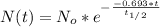
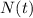 is the amount given a certain t time, and
is the amount given a certain t time, and 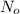 is the initial amount.
is the initial amount. 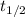 is the half life.
is the half life.