
Chemistry, 29.10.2019 04:31 AdanNava699
Acyanide solution with avolume of 12.73 ml was treated with 25.00 ml of ni2+solution (containing
excess ni2+) to convert the cyanide totetracyanonickelate(ii):
4cn- + ni2+ (cn)42-
the excess ni2+ was then titrated with 10.15 ml of0.01307 m acid
(edta). one mole of this reagent reacts with 1 mol ofni2+:
ni2+ + edta4- (edta)2-
ni(cn)42- does not react with edta. if 39.35ml of the 0.01307 m edta was required to react
with 30.10 ml of the original ni2+ solution, calculatethe molarity of cn- in the 12.73 ml cyanide
sample.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Assume that the variables x and y are inversely related. if k = 18, what is the value of y for each of the following points? be sure and record your data to be used in the following problem.
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2


Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Acyanide solution with avolume of 12.73 ml was treated with 25.00 ml of ni2+solution (containing
Questions

Mathematics, 17.02.2021 01:20

Biology, 17.02.2021 01:20


Mathematics, 17.02.2021 01:20

Mathematics, 17.02.2021 01:20












Mathematics, 17.02.2021 01:20



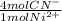 = 1,178x10⁻³ moles of CN⁻
= 1,178x10⁻³ moles of CN⁻

