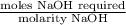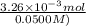Chemistry, 24.09.2019 22:20 PerksInLife
Student weighs out 0.287 g of ascorbic acid (h2ch06), a diprotic acid, into a 250. ml k and dilutes to the mark with distilled water. he plans to titrate the acid with tion olume of naoh ssolution the student will need to add to reach the final equ ur answer to 3 significant digits. ence i don't know submit | privacy oation. all rights reserved. terms of use zo19 micorw

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
Student weighs out 0.287 g of ascorbic acid (h2ch06), a diprotic acid, into a 250. ml k and dilutes...
Questions









Computers and Technology, 02.11.2019 05:31










Mathematics, 02.11.2019 05:31

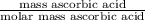
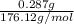
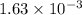 mol
mol
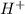 =
= 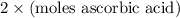

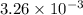 mol
mol