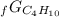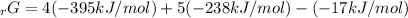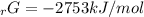Chemistry, 02.11.2019 05:31 WhiteMex69
Given the values of δgfo given below in kj/mol, calculate the value of δgo in kj for the combustion of 1 mole of butane to form carbon dioxide and liquid water. δgfo (c4h10(g)) = -17 δgfo (co2(g)) = -395 δgfo (h2o(l)) = -238

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Calculate the enthalpy of the following reaction: 4 b (s) + 3 o2 (g) → 2 b2o3 (s) given the following pertinent information: (a) b2o3 (s) + 3 h2o (g) → 3 o2 (g) + b2h6 (g), δhoa = +2035 kj (b) 2 b (s) + 3 h2 (g) → b2h6 (g), δhob = +36 kj (c) h2 (g) + latex: \frac{1}{2} 1 2 o2 (g) → h2o (l), δhoc = −285 kj (d) h2o (l) → h2o (g), δhod = +44 kj
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Given the values of δgfo given below in kj/mol, calculate the value of δgo in kj for the combustion...
Questions




English, 10.01.2022 21:50

Social Studies, 10.01.2022 21:50



English, 10.01.2022 21:50




Social Studies, 10.01.2022 21:50



Mathematics, 10.01.2022 22:00

History, 10.01.2022 22:00





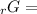 4Δ
4Δ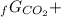 5Δ
5Δ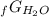 -Δ
-Δ