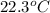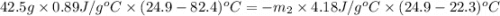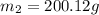Physics, 29.07.2019 02:00 christabell0303
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and dropped into a calorimeter containing water (specific heat capacity of water is 4.18 j/goc) initially at 22.3oc. the final temperature of the water is 24.9oc. calculate the mass of water in the calorimeter. ignore significant figures for this problem.

Answers: 1


Another question on Physics

Physics, 22.06.2019 10:30
Find the magnetic field a distance r from the center of a long wire that has radius a and carries a uniform current per unit area j in the positive z direction.
Answers: 2

Physics, 22.06.2019 12:50
Air is contained in a variable-load piston-cylinder device equipped with a paddle wheel. initially, air is at 400 kpa and 17°c. the paddle wheel is now turned by an external electric motor until 75 kj/kg of work has been transferred to air. during this process, heat is transferred to maintain a constant air temperature while allowing the gas volume to triple. calculate the required amount of heat transfer in kj/kg.
Answers: 2

Physics, 22.06.2019 16:20
Select the correct answer. what does the process of natural selection involve?
Answers: 1

Physics, 22.06.2019 18:00
Astudent pushes a 60-n block across the floor for a distance of 10 m. how much work was done to move the block
Answers: 1
You know the right answer?
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and...
Questions


Mathematics, 18.09.2019 08:20

English, 18.09.2019 08:20




English, 18.09.2019 08:20

Mathematics, 18.09.2019 08:20







Social Studies, 18.09.2019 08:20

Biology, 18.09.2019 08:20

Mathematics, 18.09.2019 08:20

Computers and Technology, 18.09.2019 08:30

Physics, 18.09.2019 08:30

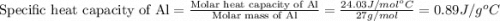
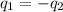
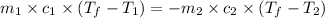
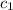 = specific heat of aluminum =
= specific heat of aluminum = 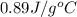
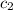 = specific heat of water =
= specific heat of water = 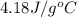
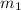 = mass of Al = 42.5 g
= mass of Al = 42.5 g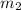 = mass of water = ?
= mass of water = ?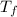 = final temperature of water =
= final temperature of water = 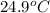
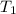 = initial temperature of Al =
= initial temperature of Al = 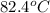
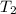 = initial temperature of water =
= initial temperature of water =