
Physics, 17.06.2021 15:10 montanolumpuy
If the outermost electron in an atom is excited to a very high energy state, its orbit is far beyond that of the other electrons. To a good approximation, we can think of the electron as orbiting a compact core with a charge equal to the charge of a single proton. The outer electron in such a Rydberg atom thus has energy levels corresponding to those of hydrogen.
Sodium is a common element for such studies. How does the radius you calculated in part A compare to the approximately 0.20 nm radius of a typical sodium atom?
r100/rNa = .

Answers: 2


Another question on Physics

Physics, 22.06.2019 05:30
Because light travels in a straight line and casts a shadow, isaac newton hypothesized that light is
Answers: 1

Physics, 22.06.2019 14:30
Which compound is held together by the electrostatic force between two ions? a. co2 b. cci4 c. h2s d. mgf2
Answers: 1

Physics, 22.06.2019 16:00
The frequency of the fundamental of the guitar string is 320 hz. at what speed v do waves move along that string?
Answers: 2

Physics, 22.06.2019 17:20
In a system with only a single force acting upon a body, what is the relationship between the change in kinetic energy and the work done by the force? answers: work is equal to the change in kinetic energy.work depends on the square of the change in potential energy.work is equal to the negative of the change in kinetic energy.work is equal to the square of the change in kinetic energy
Answers: 2
You know the right answer?
If the outermost electron in an atom is excited to a very high energy state, its orbit is far beyond...
Questions

Geography, 25.03.2020 21:00



Physics, 25.03.2020 21:01

Mathematics, 25.03.2020 21:01

History, 25.03.2020 21:01

Biology, 25.03.2020 21:01



Mathematics, 25.03.2020 21:01






English, 25.03.2020 21:01

Mathematics, 25.03.2020 21:01


Biology, 25.03.2020 21:01

Mathematics, 25.03.2020 21:01

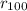 /
/ 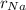 is 2645.0
is 2645.0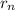 = n²α₀
= n²α₀

