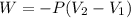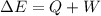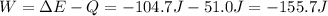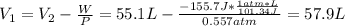Physics, 28.06.2020 02:01 Ezekielcassese
As a system increases in volume, it absorbs 51.0 J of energy in the form of heat from the surroundings. The piston is working against a pressure of 0.557 atm. The final volume of the system is 55.1 L. What was the initial volume of the system if the internal energy of the system decreased by 104.7 J

Answers: 2


Another question on Physics

Physics, 22.06.2019 05:30
Física: un futbolista patea hacia el arco con una velocidad de 15m/ah, calcular: a)el alcance para un ángulo de tiro de 25? , b) el tiempo que el balón permanece en el aire.
Answers: 3

Physics, 22.06.2019 14:20
4r-134a enters the condenser of a residential heat pump at 800 kpa and 50°c at a rate of 0.022 kg/s and leaves at 750 kpa subcooled by 3°c. the refrigerant enters the compressor at 200 kpa superheated by 4°c determine (a) the isentropic efficiency of the compressor, (b) the rate of heat supplied to the heated room, and (c) the cop of the heat pump. also, determine (d) the cop and rate of heat supplied to the heated room if this heat pump operated on the ideal vapor-compression cycle between the pressure limits of 200 and 800 kpa. (0.757, 4.37 kw, 5.12, 6.18, 3.91 kw)
Answers: 3

Physics, 22.06.2019 18:00
Consider an ideal gas at 27.0 degrees celsius and 1.00 atmosphere pressure. imagine the molecules to be uniformly spaced, with each molecule at the center of a small cube. what is the length l of an edge of each small cube if adjacent cubes touch but don't overlap?
Answers: 2

Physics, 22.06.2019 18:00
Sunidhi made a study chart about changes in states of matter. which headings best complete the chart?
Answers: 1
You know the right answer?
As a system increases in volume, it absorbs 51.0 J of energy in the form of heat from the surroundin...
Questions













Mathematics, 02.03.2020 19:26






Computers and Technology, 02.03.2020 19:26