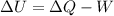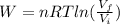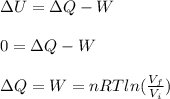An ideal monatomic gas at temperature T is held in a container. If the gas is compressed isothermally, that is at constant temperature, from a volume of Vi to Vf ,
a) What is the change in the (internal) energy of the gas?
b) How much work has been done on the gas?
c) Has heat been transferred into or out of the gas during the process? If so, how much?
d) Show that the 1st law of thermodynamics is satisfied.

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:20
Imagine you had to physically add electrons, one at a time, to a previously neutral conductor. you add one electron very easily, but the second electron requires more work. in your initial post to the discussion, explain why this is. also, what happens to the work needed to add the third, fourth, fifth, and subsequent electrons
Answers: 1

Physics, 22.06.2019 16:00
What part of the ear is names after tools, such as the hammer and the anvil?
Answers: 1

Physics, 23.06.2019 02:00
Farmer a has a deep well and needs a lot of water for her animals and crops. her neighbor, farmer b, has a shallower well. he needs less water than farmer a because his farm is smaller. in years of drought, sometimes farmer b can't get any water from his wells. what can farmer b do to decrease the likelihood of his well running dry?
Answers: 3

Physics, 23.06.2019 02:20
What are known as the properties of substances that describe a substance et does not change that substances?
Answers: 1
You know the right answer?
An ideal monatomic gas at temperature T is held in a container. If the gas is compressed isothermall...
Questions

Social Studies, 29.01.2020 03:07


Mathematics, 29.01.2020 03:07

Mathematics, 29.01.2020 03:07

Mathematics, 29.01.2020 03:07

Mathematics, 29.01.2020 03:07


Chemistry, 29.01.2020 03:07


History, 29.01.2020 03:07


Physics, 29.01.2020 03:08


Biology, 29.01.2020 03:08


Mathematics, 29.01.2020 03:08

English, 29.01.2020 03:08

Mathematics, 29.01.2020 03:08


English, 29.01.2020 03:08