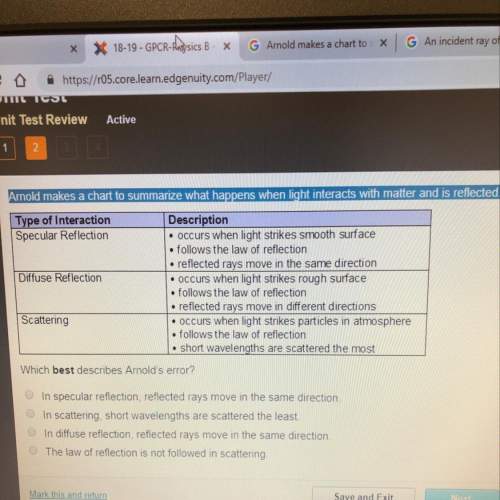
1. in a single atom, no more than 2 electrons can occupy a single orbital? a. true b. false
...

1. in a single atom, no more than 2 electrons can occupy a single orbital? a. true b. false
2. the maximum number of electrons allowed in a p sublevel of the 3rd principal level is?
a. 1 b.6 c. 2 d. 8 e. 3
3. a neutral atom has a ground state electronic configuration of 1s^2 2s^2. which of the following statements concerning this atom is/are correct? a. the atom has a total of two orbitals occupied. b. all of the above. c. the atom has no unpaired electrons. d. the atom contains 4 protons. e. the element has atomic number 4.

Answers: 3


Another question on Physics

Physics, 21.06.2019 18:00
Acommercial passenger jet can travel up to 970 km/hr at an altitude of 30,000 feet. at this speed, how long will it take the jet to travel 1,865 kilometers? a. 2.3 hrs b. 1.9 hrs c. 1.7 hrs d. 1.5 hrs
Answers: 1

Physics, 22.06.2019 08:30
You win the lottery and decide to impress your friends by exhibiting a million-dollar cube of gold. at the time, gold is selling for $ 426.60 per troy ounce, and 1.0000 troy ounce equals 31.1035 g. -how tall would your million-dollar cube be? en cm
Answers: 2

Physics, 22.06.2019 14:30
What conclusion can be made based on the temperature of soil when the light hits the soil at 0°, 45°, and 90° angles in section 2 of the experiment? did your results support your hypothesis? why or why not?
Answers: 1

Physics, 22.06.2019 18:00
Aprisoner is forced to go into one of three rooms, but he can choose which room. the first room is ablaze with fire. the second one is rigged with explosives that will go off as soon as he enters. the third contains a pair of lions who haven't eaten in years. which room should he choose to survive?
Answers: 2
You know the right answer?
Questions



Mathematics, 29.12.2019 03:31

Biology, 29.12.2019 03:31

Mathematics, 29.12.2019 03:31

Health, 29.12.2019 03:31

Mathematics, 29.12.2019 03:31

Mathematics, 29.12.2019 03:31

History, 29.12.2019 03:31



Mathematics, 29.12.2019 03:31


French, 29.12.2019 03:31

Mathematics, 29.12.2019 03:31

Mathematics, 29.12.2019 03:31

Mathematics, 29.12.2019 03:31

Mathematics, 29.12.2019 03:31

Mathematics, 29.12.2019 03:31

Arts, 29.12.2019 03:31

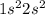 .
.