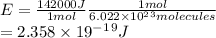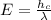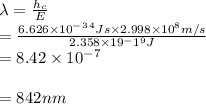Answers: 3


Another question on Physics

Physics, 22.06.2019 11:20
More solar radiation is absorbed by earth’s surface than by
Answers: 1

Physics, 22.06.2019 11:30
Which of the following is the phase that results when the moon is on the opposite side of the earth from the sun? a. quarter moon b. crescent moon c. new moon d. full moon
Answers: 1

Physics, 22.06.2019 15:00
Astudent throws a water balloon with speed v0 from a height h = 1.76 m at an angle θ = 21° above the horizontal toward a target on the ground. the target is located a horizontal distance d = 9.5 m from the student’s feet. assume that the balloon moves without air resistance. use a cartesian coordinate system with the origin at the balloon's initial position. (a) what is the position vector, rtarge t, that originates from the balloon's original position and terminates at the target? put this in terms of h and d, and represent it as a vector using i and j. (b) in terms of the variables in the problem, determine the time, t, after the launch it takes the balloon to reach the target. your answer should not include h. (c) create an expression for the balloon's vertical position as a function of time, y(t), in terms of t, vo, g, and θ. (d) determine the magnitude of the balloon's initial velocity, v0, in meters per second, by eliminating t from the previous two expressions.
Answers: 3

Physics, 22.06.2019 21:30
Complete the sentence to describe the law of conservation of energy. the law of conservation of energy states that energy cannot be created
Answers: 3
You know the right answer?
Hydrogen peroxide is sold commercially as an aqueous solution in brown bottles to protect it from li...
Questions

Computers and Technology, 08.12.2019 15:31

Computers and Technology, 08.12.2019 15:31





Biology, 08.12.2019 15:31


English, 08.12.2019 15:31



Biology, 08.12.2019 15:31

Arts, 08.12.2019 15:31


Biology, 08.12.2019 15:31