
Physics, 08.12.2019 15:31 dominickstrickland
The sugar molecule known as sucrose is a polar molecule. which statement best summarizes how sucrose will dissolve in water?
a) water and sucrose dissolve readily because of the differences in particle size.
b) sucrose is not soluble in water since neither molecule is a gas at room temperature.
c) since water and sucrose are both polar molecules, sucrose will dissolve readily in water.
eliminate
d) since water is a non-polar molecule, it will dissolve sucrose readily since unlike molecules are more soluble.

Answers: 3


Another question on Physics

Physics, 21.06.2019 22:50
If the temperature were raised very high, classically what would we expect the heat capacity per object to be for this one-dimensional system? give a numerical value. chigh t = __ j/k/object (one reason for the discrepancy is that the high-temperature limit assumes that the number of oscillators is large (n > > 1), which is not the case in this tiny system.)
Answers: 2

Physics, 21.06.2019 23:30
Edward tolman explained the results of his study by theorizing that the rats were learning about the maze during every trial but they a. were agitated because other groups were getting reinforcement b. could not remember how to demonstrate it without reinforcement c. were not motivated to demonstrate it without reinforcement d. seemed to be too lazy to actually work without reinforcement
Answers: 3

Physics, 22.06.2019 13:10
Which of the following are used in an electrochemical cell? a. electrodes b. electrolyte c. terminals d. all of the above
Answers: 3

Physics, 23.06.2019 02:30
Ajet plane flying 600 m/s experience an acceleration of 4.0 g when pulling out of a circular dive. what is the radius of curvature part of the path in which the plane is flying? a) 7100 m b) 650 m c) 9200 m d) 1200 m
Answers: 3
You know the right answer?
The sugar molecule known as sucrose is a polar molecule. which statement best summarizes how sucrose...
Questions




Mathematics, 02.10.2019 18:10

English, 02.10.2019 18:10

World Languages, 02.10.2019 18:10



English, 02.10.2019 18:10


Mathematics, 02.10.2019 18:10



Computers and Technology, 02.10.2019 18:10






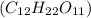 is a polar molecule as there is oxygen and hydrogen bond which will make oxygen atom partially negative and hydrogen partially positive.
is a polar molecule as there is oxygen and hydrogen bond which will make oxygen atom partially negative and hydrogen partially positive.

