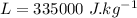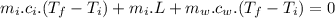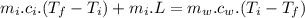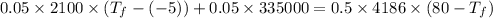Physics, 18.02.2020 02:22 cherylmorton7302
In an insulated container, 0.50 kg of water at 80°C is mixed with 0.050 kg of ice at −5.0°C. After a while, all the ice melts, leaving only the water. Find the final temperature of the water. The freezing point of water is 0°C.

Answers: 1


Another question on Physics

Physics, 22.06.2019 08:30
Researchers determine that the biodiversity in a woodland region is declining. they identify two major threats to the region's biodiversity, a method to address each threat, and the expected outcome of each method. this information is shown in the table. a) reforestation would not benefit many species because most forest species live on the ground. b) reforestation would take the longest time to be effective because trees take several years to grow. c) biological augmentation would benefit only a few species because is is typically not very effective. d) biological augmentation would take less time to ve effective because it targets the majority of prey species.
Answers: 3

Physics, 22.06.2019 11:00
What is the measurement of how fast an object moves relative to a reference point?
Answers: 1


Physics, 22.06.2019 23:40
Consider a u-tube whose arms are open to the atmosphere. now equal volumes of water and light oil (ρ = 49.3 lbm/ft3) are poured from different arms. a person blows from the oil side of the u-tube until the contact surface of the two fluids moves to the bottom of the u-tube, and therefore, the liquid levels in the two arms are the same. if the fluid height in each arm is 30 in, determine the gage pressure exerted by the person on the oil by blowing.
Answers: 3
You know the right answer?
In an insulated container, 0.50 kg of water at 80°C is mixed with 0.050 kg of ice at −5.0°C. After a...
Questions

Computers and Technology, 01.05.2021 23:50



Mathematics, 01.05.2021 23:50

Mathematics, 01.05.2021 23:50

Mathematics, 01.05.2021 23:50





Mathematics, 01.05.2021 23:50

Social Studies, 01.05.2021 23:50



Mathematics, 01.05.2021 23:50


Mathematics, 01.05.2021 23:50

Biology, 01.05.2021 23:50


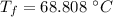
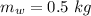 initial temperature of water,
initial temperature of water, 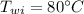 mass of ice,
mass of ice, 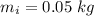 initial temperature of ice,
initial temperature of ice, 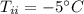 Specific heat capacity of water,
Specific heat capacity of water, 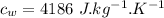 Specific heat capacity of ice,
Specific heat capacity of ice, 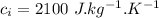 Latent heat of fusion of ice,
Latent heat of fusion of ice,