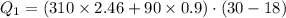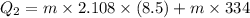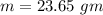Physics, 14.02.2020 19:52 chanavictor2747
Suppose 310. grams of ethanol (ethyl alcohol) is in an aluminum cup of 90.0 grams. Both of these are at 30.0C. A mass m of ice at – 8.5C is taken from a freezer and added to the alcohol in the cup. The final temperature of all the components is 18.0C. Assuming no heat was lost from the system, calculate the mass m of the ice added.

Answers: 2


Another question on Physics

Physics, 22.06.2019 00:30
There are weak or strong attractive forces between atoms of liquids with a high viscosity
Answers: 3

Physics, 22.06.2019 11:30
If we relied solely on the nonrenewable resources found in the u.s., which one would we run out of first at current usage levels? a. natural gas b. oil c. uranium d. coal
Answers: 1

Physics, 22.06.2019 12:30
Hydrogen atoms are excited by a laser to the =4n=4 state and then allowed to emit. what is the maximum number of distinct emission spectral lines (lines of different wavelengths) that can be observed from this system? 8 6 2 7 4 5 1 3 calculate the wavelength of the 4⟶14⟶1 transition. =λ=
Answers: 2

Physics, 22.06.2019 17:40
Which component of the earth’s atmosphere is decreased due to photosynthesis?
Answers: 1
You know the right answer?
Suppose 310. grams of ethanol (ethyl alcohol) is in an aluminum cup of 90.0 grams. Both of these are...
Questions

Mathematics, 07.02.2021 05:10

Social Studies, 07.02.2021 05:10






Mathematics, 07.02.2021 05:10


Mathematics, 07.02.2021 05:10

History, 07.02.2021 05:20



Computers and Technology, 07.02.2021 05:20


Mathematics, 07.02.2021 05:20

Mathematics, 07.02.2021 05:20


Biology, 07.02.2021 05:20

Mathematics, 07.02.2021 05:20


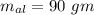
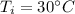

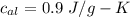

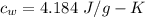
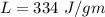
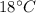 is reached
is reached