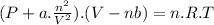Answers: 3


Another question on Physics

Physics, 22.06.2019 02:30
If a refrigerator is a heat pump that follows the first law of thermodynamics, how much heat was removed from food inside of the refrigerator if it released 380j of energy to the room?
Answers: 1

Physics, 22.06.2019 13:00
Discuss how the hardness or softness of the landing surface is related to the time required to stop the egg
Answers: 1

Physics, 22.06.2019 17:30
Students in an introductory physics lab are performing an experiment with a parallel-plate capacitor made of two circular aluminum plates, each 11 cm in diameter, separated by 1.0 cm. how much charge can be added to each of the plates before a spark jumps between the two plates? for such flat electrodes, assume that value of 3×106n/c of the field causes a spark.
Answers: 2

Physics, 22.06.2019 22:40
Calculate the volume of this regular solid. what is the volume of the cone? round your answer to the nearest hundredth
Answers: 1
You know the right answer?
A9.800 mol sample of nitrogen gas is maintained in a 0.8166 l container at 301.8 k. what is the pres...
Questions








Advanced Placement (AP), 08.01.2020 06:31






History, 08.01.2020 06:31


Mathematics, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31