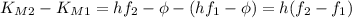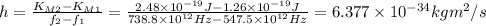When light with a frequency f1 = 547.5 thz illuminates a metal surface, the most energetic photoelectrons have 1.260 x 10^-19 j of kinetic energy. when light with a frequency f2 = 738.8 thz is used instead, the most energetic photo-electrons have 2.480 x 10^-19 j of kinetic energy
using these experimental results, determine the approximate value of planck's constant.
express your answer using four significant figures.

Answers: 3


Another question on Physics


Physics, 21.06.2019 17:30
What produces light and all other electromagnetic waves? a) the photoelectric phenomena b) heated metals c) charged particles d) radioactive samples (pls explain if you can : )
Answers: 1

Physics, 22.06.2019 03:50
Two polarizers are oriented at 70∘∘ to one another. unpolarized light falls on them. what fraction of the light intensity is transmitted?
Answers: 1

Physics, 22.06.2019 07:50
Determine the fraction of the magnitude of kinetic energy lost by a neutron (m1 = 1.01 u) when it collides head-on and elastically with a target particle at rest which is 21h (heavy hydrogen, m = 2.01 u).
Answers: 3
You know the right answer?
When light with a frequency f1 = 547.5 thz illuminates a metal surface, the most energetic photoelec...
Questions

Computers and Technology, 05.05.2020 07:06




Geography, 05.05.2020 07:06



Biology, 05.05.2020 07:06

Mathematics, 05.05.2020 07:06

Mathematics, 05.05.2020 07:06


Mathematics, 05.05.2020 07:06

Social Studies, 05.05.2020 07:06





History, 05.05.2020 07:06

Arts, 05.05.2020 07:06

History, 05.05.2020 07:06

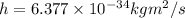
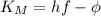 .
.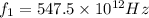 we get
we get 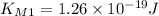 and for
and for 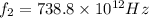 we get
we get 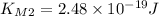 , so we have:
, so we have: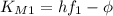
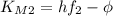
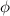 by substracting the first equation to the second:
by substracting the first equation to the second: