
A25.0-g sample of copper at 363 k is placed in i 00.0 g of water at 293 k. the copper and water quickly come to the sa me temperature by the process of heat transfer from copper to water. calculate the final temperature of the water. the molar heat capacity of copper is 24.5 j · k-1 ·mol-1 and that of water is 75.2 1 · k -1 -mol-1•

Answers: 2


Another question on Physics

Physics, 22.06.2019 16:00
Which composition of water moves to begin a deepwater current?
Answers: 2



Physics, 23.06.2019 08:10
Which of the following situations would produce an average velocity of zero? o a. a horse galloping in a field b. a round-trip to school and back o c. a trip, first to the moon, then onto mars o d. the criss-crossing path of a flying bug
Answers: 2
You know the right answer?
A25.0-g sample of copper at 363 k is placed in i 00.0 g of water at 293 k. the copper and water quic...
Questions


Mathematics, 03.07.2019 15:00



Social Studies, 03.07.2019 15:00

Spanish, 03.07.2019 15:00



Social Studies, 03.07.2019 15:00


History, 03.07.2019 15:00







English, 03.07.2019 15:00

Social Studies, 03.07.2019 15:00

Mathematics, 03.07.2019 15:00

 ×mass×ΔT
×mass×ΔT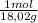 ×mass×ΔT
×mass×ΔT

