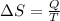Physics, 28.10.2019 08:31 Frenchfries13
Quantity a of an ideal gas is at absolute temperature t, and a second quantity b of the same gas is at absolute temperature 2t. heat is added to each gas, and both gases are allowed to expand isothermally. if both gases were initially at the same absolute temperature, would they still undergo the same entropy change? no, gas a would undergo the greater entropy change. no, gas b would undergo the greater entropy change. yes, both gases would have the same entropy.

Answers: 1


Another question on Physics

Physics, 22.06.2019 16:30
Is there a point between a 10 nc charge and a 20 nc charge at which the electric field is zero?
Answers: 2

Physics, 22.06.2019 17:00
Air conditioners not only cool air, but dry it as well. suppose that a room in a home measures 5.0m×9.0m×2.4m . if the outdoor temperature is 30 ∘c and the vapor pressure of water in the air is 85 % of the vapor pressure of water at this temperature, what mass of water must be removed from the air each time the volume of air in the room is cycled through the air conditioner? the vapor pressure for water at 30 ∘c is 31.8 torr.
Answers: 3

Physics, 23.06.2019 00:00
Boiling water is an example of physical change. true or false
Answers: 2

Physics, 23.06.2019 05:00
Heating water for hot cocoa while sitting next to a campfire can be examples of conduction, convenction and radiation at the same time agree disagree it depends how you know
Answers: 2
You know the right answer?
Quantity a of an ideal gas is at absolute temperature t, and a second quantity b of the same gas is...
Questions

Mathematics, 16.10.2019 14:00

Mathematics, 16.10.2019 14:00

Physics, 16.10.2019 14:00




Spanish, 16.10.2019 14:00


Mathematics, 16.10.2019 14:00




Computers and Technology, 16.10.2019 14:00





Business, 16.10.2019 14:00