Mathematics, 05.07.2021 14:00 kmchippps
Calculate the pH of a buffer solution made by mixing 300 mL of 0.2 M acetic acid, CH3COOH, and 200 mL of 0.3 M of its salt sodium acetate, CH3COONa, to make 500 mL of solution. Ka for CH3COOH = 1.76×10–5

Answers: 3


Another question on Mathematics


Mathematics, 21.06.2019 23:00
How many heads would you expect if you flipped a coin twice? first, fill in the table below with the correct probabilities. hint: the sample space for flipping a coin twice is {hh, ht, th, tt}. a = b = c =
Answers: 3

Mathematics, 22.06.2019 00:20
Which of the following is equal to the square root of the cube root of 5 ? (1 point) 5 to the power of 1 over 3 5 to the power of 1 over 6 5 to the power of 2 over 3 5 to the power of 3 over 2
Answers: 1

Mathematics, 22.06.2019 01:00
The collection of beautiful oil paintings currently on display at an art gallery well defined; set not well defined; not a set
Answers: 2
You know the right answer?
Calculate the pH of a buffer solution made by mixing 300 mL of 0.2 M acetic acid, CH3COOH, and 200 m...
Questions

Business, 21.09.2019 14:30





Mathematics, 21.09.2019 14:30


Mathematics, 21.09.2019 14:30




Geography, 21.09.2019 14:30


Biology, 21.09.2019 14:30




World Languages, 21.09.2019 14:30

Mathematics, 21.09.2019 14:30

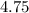 .
. 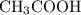 and
and 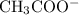 are equal.
are equal. 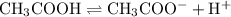
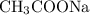 is a salt soluble in water. Once in water, it would readily ionize to give
is a salt soluble in water. Once in water, it would readily ionize to give 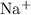 ions.
ions. 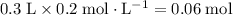 of
of 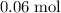 of
of 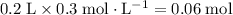 of
of 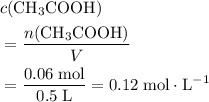 .
.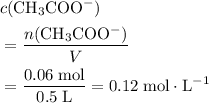 .
.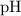 of this buffer solution would be the same as the
of this buffer solution would be the same as the 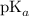 of
of 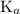 :
: .
.