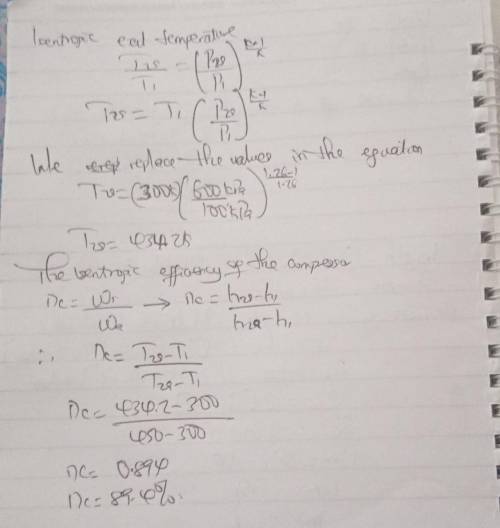Engineering, 27.08.2020 06:01 sweetieval17oz0f8q
Carbon dioxide enters an adiabatic compressor at 100 kPa and 300 K at a rate of 1.8 kg/s and exits at 600 kPa and 450 K. Neglecting the kinetic energy changes, Determine: (a) The isentropic efficiency of the compressor.

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
Apump is used to circulate hot water in a home heating system. water enters the well-insulated pump operating at steady state at a rate of 0.42 gal/min. the inlet pressure and temperature are 14.7 lbf/in.2, and 180°f, respectively; at the exit the pressure is 60 lbf/in.2 the pump requires 1/15 hp of power input. water can be modeled as an incompressible substance with constant density of 60.58 lb/ft3 and constant specific heat of 1 btu/lb or. neglecting kinetic and potential energy effects, determine the temperature change, in °r, as the water flows through the pump.
Answers: 1

Engineering, 04.07.2019 18:20
Inadequate stores control is not an obstacle to effective work order system. (clo4) a)-true b)-false
Answers: 3

Engineering, 04.07.2019 18:20
Derive the correction factor formula for conical nozzle i=-(1+ cosa) and calculate the nozzle angle correction factor for a nozzle whose divergence hal-fangle is 13 (hint: assume that all the mass flow originates at the apex of the cone.
Answers: 3

Engineering, 04.07.2019 18:20
Asolid cylinder is concentric with a straight pipe. the cylinder is 0.5 m long and has an outside diameter of 8 cm. the pipe has an inside diameter of 8.5 cm. the annulus between the cylinder ad the pipe contains stationary oil. the oil has a specific gravity of 0.92 and a kinematic viscosity of 5.57 x 10-4 m2/s. most nearly, what is the force needed to move the cylinder along the pipe at a constant velocity of 1 m/s?
Answers: 3
You know the right answer?
Carbon dioxide enters an adiabatic compressor at 100 kPa and 300 K at a rate of 1.8 kg/s and exits a...
Questions







Computers and Technology, 11.02.2020 17:29

Social Studies, 11.02.2020 17:29




Social Studies, 11.02.2020 17:30