
Engineering, 07.04.2020 03:16 mimi5937
Applying the Entropy Balance: Closed Systems Five kg of carbon dioxide (CO2) gas undergoes a process in a well-insulated piston–cylinder assembly from 2 bar, 280 K to 20 bar, 520 K. If the carbon dioxide behaves as an ideal gas, determine the amount of entropy produced, in kJ/K, assuming (a) constant specific heats with c= = 0.939 kJ/kg · K. (b) variable specific heats. Compare the results of parts (a) and (b).

Answers: 2


Another question on Engineering

Engineering, 04.07.2019 18:10
An air conditioning system consist of a 5 cm diameter pipe, operating at a pressure of 200 kpa. the air initially enters the pipe at 15°c with a velocity of 20 m/s and relative humidity of 80%. if the heat supply throughout the process is 960 w, determine the relative humidity and the temperature at the outlet
Answers: 3

Engineering, 04.07.2019 18:10
Water at 70°f and streams enter the mixing chamber at the same mass flow rate, determine the temperature and the quality of the exiting stream. 0 psia is heated in a chamber by mixing it with saturated water vapor at 20 psia. if both streams enters the mixing chamber at the same mass flow rate, determine the temperature and the quality of the existing system.
Answers: 2

Engineering, 04.07.2019 18:10
Different types of steels contain different elements that alter the characteristics of the steel. for each of the following elements, explain what the element does when alloyed with steel.
Answers: 2

Engineering, 04.07.2019 18:10
Water in a partially filled large tank is to be supplied to the roof top, which is 8 m above the water level in the tank, through a 2.2-cm-internal-diameter pipe by maintaining a constant air pressure of 300 kpa (gage) in the tank. if the head loss in the piping is 2 m of water, determine the discharge rate of the supply of water to the roof top in liters per second.
Answers: 3
You know the right answer?
Applying the Entropy Balance: Closed Systems Five kg of carbon dioxide (CO2) gas undergoes a process...
Questions

History, 18.10.2020 15:01

Computers and Technology, 18.10.2020 15:01


History, 18.10.2020 15:01


Arts, 18.10.2020 15:01


History, 18.10.2020 15:01


Mathematics, 18.10.2020 15:01

Advanced Placement (AP), 18.10.2020 15:01


Mathematics, 18.10.2020 15:01


Mathematics, 18.10.2020 15:01

English, 18.10.2020 15:01

History, 18.10.2020 15:01

Chemistry, 18.10.2020 15:01


Mathematics, 18.10.2020 15:01

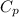 = 0.939 kJ/kg.K , m = 5 kg , T₂ = 520 K , T₁ = 280
= 0.939 kJ/kg.K , m = 5 kg , T₂ = 520 K , T₁ = 280![m[c_p ln(\frac{T_2}{T_1}) - Rln(\frac{P_2}{P_1})]](/tpl/images/0585/8806/1d12a.png)
![5[(0.939) ln(\frac{520}{280}) - (\frac{8.314}{44.01})ln(\frac{20}{2})]](/tpl/images/0585/8806/340b4.png)
![m[\frac{s^0(T_2) - s^0(T_1)}{M} - Rln(\frac{P_2}{P_1})]](/tpl/images/0585/8806/932df.png)
![5[\frac{236.575 - 211.376}{44.01} - (\frac{8.314}{44.01})ln(\frac{20}{2})]](/tpl/images/0585/8806/335ff.png)


