
Chemistry, 18.10.2020 15:01 chaparro0512
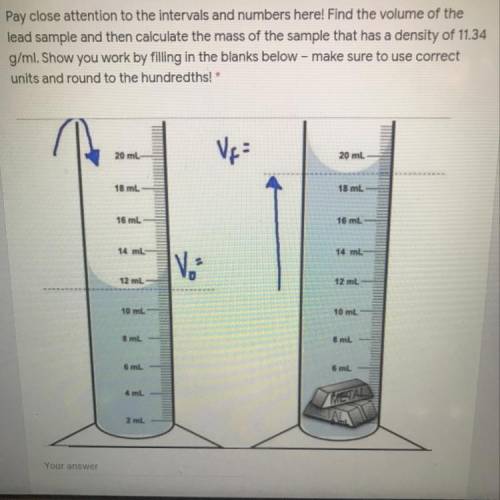
Pay close attention to the intervals and numbers here! Find the volume of the
lead sample and then calculate the mass of the sample that has a density of 11.34
g/ml. Show you work by filling in the blanks below - make sure to use correct
units and round to the hundredths!*
VA
20 ml
20 ml
18 ml
18 ml
16 ml.
16 mL
14 mL
14 ml
V.
12 ml
12 ml
10 ml
10 ml
8 ml
8 ml
6 ml
4 ml
USTA
2 ml

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
Pay close attention to the intervals and numbers here! Find the volume of the
lead sample and then...
Questions

Mathematics, 09.12.2020 03:50

Mathematics, 09.12.2020 03:50


History, 09.12.2020 03:50

History, 09.12.2020 03:50

Mathematics, 09.12.2020 03:50


Mathematics, 09.12.2020 03:50


History, 09.12.2020 03:50

English, 09.12.2020 03:50





Mathematics, 09.12.2020 03:50

Mathematics, 09.12.2020 03:50

History, 09.12.2020 03:50

English, 09.12.2020 03:50

Mathematics, 09.12.2020 03:50



