Engineering, 21.02.2020 03:59 katie713518
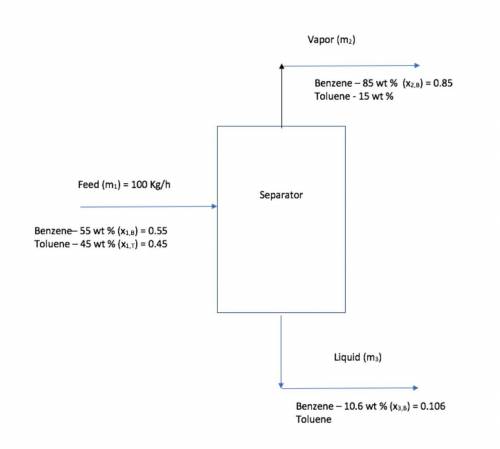
A steady state and continuous separator has a total feed rate of 100. kg/h of a 55.0 wt. % benzene mixture. The balance is toluene (i. e., 45 wt. % toluene). A vapor stream leaves the process and has a benzene concentration of 85.0% by mass and a liquid stream leaving the process has a benzene concentration of 10.6% by mass. a. Draw and label the process flow chart. Develop a consistent variable naming system. b. Simplify the general balance equation. c. Write and solve the total mass balance and the benzene mass balance.

Answers: 3


Another question on Engineering

Engineering, 03.07.2019 15:10
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1

Engineering, 04.07.2019 18:10
Fluids at rest possess no flow energy. a)- true b)- false
Answers: 3

Engineering, 04.07.2019 18:10
Slip occurs via two partial dislocations because of (a) the shorter path of the partial dislocation lines; (b) the lower energy state through partial dislocations; (c) the charge balance.
Answers: 1

Engineering, 04.07.2019 18:20
Air is compressed isentropically from an initial state of 300 k and 101 kpa to a final temperature of 1000 k. determine the final pressure using the following approaches: (a) approximate analysis (using properties at the average temperature) (b) exact analysis
Answers: 1
You know the right answer?
A steady state and continuous separator has a total feed rate of 100. kg/h of a 55.0 wt. % benzene m...
Questions

Mathematics, 04.03.2020 23:29

History, 04.03.2020 23:29


History, 04.03.2020 23:29





Mathematics, 04.03.2020 23:29






Geography, 04.03.2020 23:29

Advanced Placement (AP), 04.03.2020 23:29

Advanced Placement (AP), 04.03.2020 23:29

Mathematics, 04.03.2020 23:29

History, 04.03.2020 23:29

Computers and Technology, 04.03.2020 23:29