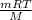Many gases are shipped in high-pressure containers. consider a steel tank whose volume is 55.0 gallons that contains o2 gas at a pressure of 16,500 kpa at 23 °c. (a) what mass of o2 does the tank contain? (b) what volume would the gas occupy at stp? (c) at what temperature would the pressure in the tank equal 150.0 atm? (d) what would be the pressure of the gas, in kpa, if it were transferred to a container at 24 °c whose volume is 55.0 l?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Many gases are shipped in high-pressure containers. consider a steel tank whose volume is 55.0 gallo...
Questions


Social Studies, 27.08.2019 18:30






Social Studies, 27.08.2019 18:30




Mathematics, 27.08.2019 18:30

Computers and Technology, 27.08.2019 18:30



Social Studies, 27.08.2019 18:30


Social Studies, 27.08.2019 18:30