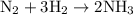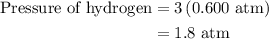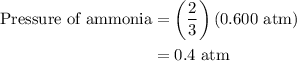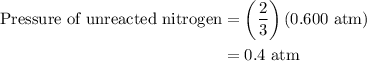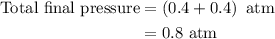Chemistry, 30.07.2019 22:00 tiffanibell71
Arigid vessel at constant temperature initially contains 0.600 atm nitrogen gas and 0.600 atm hydrogen gas. if these gases react to form ammonia and the reaction goes to completion, which choice is closest to the final total pressure after the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
Arigid vessel at constant temperature initially contains 0.600 atm nitrogen gas and 0.600 atm hydrog...
Questions




Mathematics, 24.04.2021 05:40






Mathematics, 24.04.2021 05:40

Advanced Placement (AP), 24.04.2021 05:40

Mathematics, 24.04.2021 05:40

Mathematics, 24.04.2021 05:40




History, 24.04.2021 05:40

History, 24.04.2021 05:40

Physics, 24.04.2021 05:40

English, 24.04.2021 05:40

 .
.