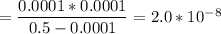Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Calculate the acid dissociation constant of a weak monoprotic acid if a 0.5m solution of this acid g...
Questions


History, 01.09.2019 07:20


Biology, 01.09.2019 07:20

English, 01.09.2019 07:20


Computers and Technology, 01.09.2019 07:20

History, 01.09.2019 07:20





Spanish, 01.09.2019 07:20

Mathematics, 01.09.2019 07:20

Mathematics, 01.09.2019 07:20

Mathematics, 01.09.2019 07:20


English, 01.09.2019 07:20


Mathematics, 01.09.2019 07:20

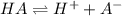
![K_a= \dfrac{[H^+][A^-]}{[HA]}](/tpl/images/0155/5739/a4583.png)
![0.5M=[A^-]+[HA]](/tpl/images/0155/5739/14b10.png)
![[HA]=0.5M-[A^-]](/tpl/images/0155/5739/ce8c0.png)
![K_a= \dfrac{[H^+][A^-]}{0.5M-[A^-]}](/tpl/images/0155/5739/a476b.png)