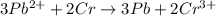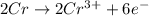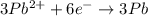Given the balanced ionic equation:
3pb2+ + 2cr ==> 3pb + 2cr3+
what is the number o...

Chemistry, 20.08.2019 11:30 mathman783
Given the balanced ionic equation:
3pb2+ + 2cr ==> 3pb + 2cr3+
what is the number of moles of electrons gained by 3.0 moles of lead ions?
(1) 5.0 mol (3)3.0 mol
(2) 2.0 mol (4) 6.0mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1
You know the right answer?
Questions

History, 10.07.2019 05:30











History, 10.07.2019 05:30




Biology, 10.07.2019 05:30


Biology, 10.07.2019 05:30


Mathematics, 10.07.2019 05:30