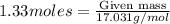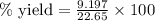Chemistry, 01.08.2019 21:30 05leslun42715
Ammonia (nh3) can be produced by the reaction of hydrogen gas with nitrogen gas: 3h2 + n2 = 2nh3 a chemist reacts 2.00 mol h2 with excess n2. the reaction yields 0.54 mol nh3. what is the percent yield of the reaction? a) 25% b) 40% c) 60% d) 80%

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Ammonia (nh3) can be produced by the reaction of hydrogen gas with nitrogen gas: 3h2 + n2 = 2nh3 a...
Questions




Computers and Technology, 14.02.2020 22:02



English, 14.02.2020 22:03



Mathematics, 14.02.2020 22:04

Mathematics, 14.02.2020 22:04









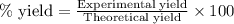 ....(1)
....(1)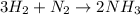
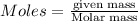 ....(2)
....(2)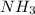 = 17.031g/mol
= 17.031g/mol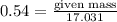
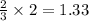 moles of ammonia.
moles of ammonia.