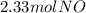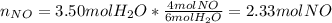`suppose you were tasked with producing some nitrogen monoxide (a. k.a. nitric oxide). i'm sure this is often requested of you. you can do it by combusting ammonia (be the equation would be as follows: 4nh3 + 5o 2→ 4no + 6h2o. if you form 3.50 mol of water, how much no forms? a. 2.33 mol. b. 4.00 mol. c. 4.50 mol. d. 6.75 mol. e. none of the above

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1
You know the right answer?
`suppose you were tasked with producing some nitrogen monoxide (a. k.a. nitric oxide). i'm sure this...
Questions


Mathematics, 19.10.2020 18:01

Computers and Technology, 19.10.2020 18:01

History, 19.10.2020 18:01

Mathematics, 19.10.2020 18:01



Mathematics, 19.10.2020 18:01


Mathematics, 19.10.2020 18:01

English, 19.10.2020 18:01

Computers and Technology, 19.10.2020 18:01







English, 19.10.2020 18:01