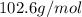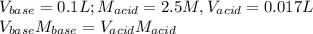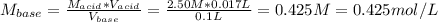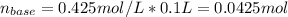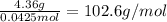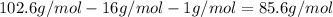Chemistry, 13.07.2019 10:20 Kennethabrown09
A4.36 g sample of an unknown alkali metal hydroxide is dissolved in 100.0 ml of water. an acid-base indicator is added and the resulting solution is titrated with 2.50 m hcl (aq) solution. the indicator changes color signaling that the equivalent point has been reached after 17.0 ml of the hydrochloric acid solution has been added. (a) what is the molar mass of the metal hydroxide? (b) what is the identity of the metal cation?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
A4.36 g sample of an unknown alkali metal hydroxide is dissolved in 100.0 ml of water. an acid-base...
Questions

Mathematics, 11.05.2021 18:40


English, 11.05.2021 18:40





Mathematics, 11.05.2021 18:40



Biology, 11.05.2021 18:40

Computers and Technology, 11.05.2021 18:40



Mathematics, 11.05.2021 18:40

English, 11.05.2021 18:40


Mathematics, 11.05.2021 18:40

Mathematics, 11.05.2021 18:40