
Chemistry, 13.03.2022 01:00 samanthasheets8006
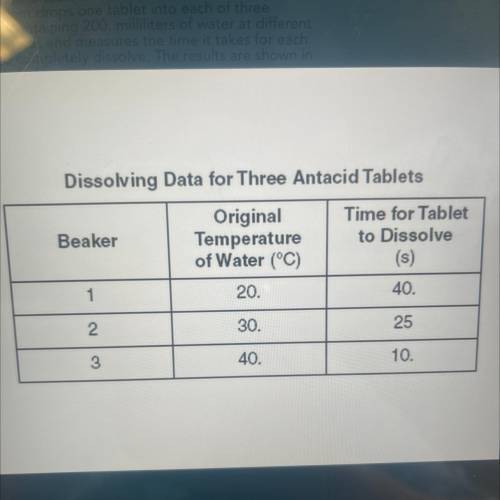
A student conducts an experiment to determine how
the temperature of water affects the rate at which an
antacid tablet dissolves in the water. The student has
three antacid tablets of the same size and composition.
The student drops one tablet into each of three
beakers containing 200. milliliters of water at different
temperatures and measures the time it takes for each
tablet to completely dissolve. The results are shown in
the table below.
10.) What change, other than temperature, would
affect the rate of dissolving?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

You know the right answer?
A student conducts an experiment to determine how
the temperature of water affects the rate at whi...
Questions

History, 20.10.2019 13:50


History, 20.10.2019 13:50

History, 20.10.2019 13:50


Mathematics, 20.10.2019 13:50

Mathematics, 20.10.2019 13:50


English, 20.10.2019 13:50


Mathematics, 20.10.2019 13:50



Mathematics, 20.10.2019 13:50

History, 20.10.2019 13:50

Mathematics, 20.10.2019 13:50

English, 20.10.2019 13:50


English, 20.10.2019 13:50



