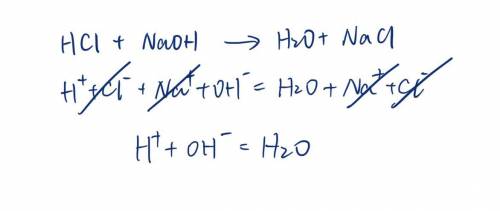Chemistry, 13.02.2022 23:50 ralphmillerrr
. A strong acid, HCl, is titrated with a strong base, NaOH. Write the net ionic equation for the reaction. Do not include spectator ions in the equation.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 21.06.2019 18:00
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1


Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1
You know the right answer?
. A strong acid, HCl, is titrated with a strong base, NaOH. Write the net ionic equation for the rea...
Questions

History, 12.02.2021 22:50

Mathematics, 12.02.2021 22:50

Mathematics, 12.02.2021 22:50




Mathematics, 12.02.2021 22:50





Mathematics, 12.02.2021 22:50


Mathematics, 12.02.2021 22:50

Mathematics, 12.02.2021 22:50

Mathematics, 12.02.2021 22:50

Mathematics, 12.02.2021 22:50


Mathematics, 12.02.2021 22:50

Arts, 12.02.2021 22:50