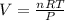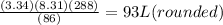Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

You know the right answer?
A sample of Ammonia gas at 650mmHg and 15 degree has a mass of 56.8g. calculate the volume occupied...
Questions

Biology, 16.04.2021 20:30

Chemistry, 16.04.2021 20:30

History, 16.04.2021 20:30

Arts, 16.04.2021 20:30

Mathematics, 16.04.2021 20:30



Mathematics, 16.04.2021 20:30

Physics, 16.04.2021 20:30



Mathematics, 16.04.2021 20:30

Mathematics, 16.04.2021 20:30

Mathematics, 16.04.2021 20:30

Mathematics, 16.04.2021 20:30



Mathematics, 16.04.2021 20:30


Mathematics, 16.04.2021 20:30

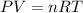 ).
). 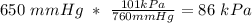
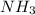 ) = (1 x 3) + (14) = 17g/mol
) = (1 x 3) + (14) = 17g/mol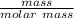
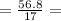 3.34 mol
3.34 mol