
Chemistry, 02.10.2021 22:00 alexandrafaber93061
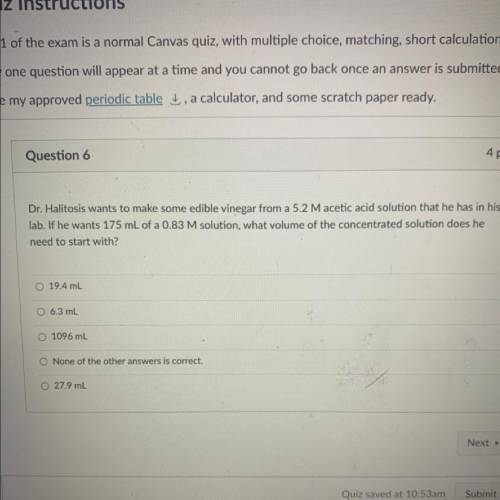
Dr. Halitosis wants to make some edible vinegar from a 5.2 Macetic acid solution that he has in his
lab. If he wants 175 mL of a 0.83 M solution, what volume of the concentrated solution does he
need to start with?
O 19.4 mL
O 6.3 mL
O1096 mL
O None of the other answers is correct.
0 27.9 mL

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Dr. Halitosis wants to make some edible vinegar from a 5.2 Macetic acid solution that he has in his...
Questions



Mathematics, 19.09.2019 07:30



History, 19.09.2019 07:30

English, 19.09.2019 07:30



Mathematics, 19.09.2019 07:30

Biology, 19.09.2019 07:30

Social Studies, 19.09.2019 07:30


English, 19.09.2019 07:30


Geography, 19.09.2019 07:30


Mathematics, 19.09.2019 07:30


Social Studies, 19.09.2019 07:30



