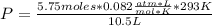A chemist is preparing to carry out a reaction that requires 5.75 moles of hydrogen gas. The chemist pumps the hydrogen into a 10.5 L rigid steel container at 20.0 °C. To what pressure, in kPa, must the hydrogen be compressed? (Show all work for full credit and circle your final answer) *

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

You know the right answer?
A chemist is preparing to carry out a reaction that requires 5.75 moles of hydrogen gas. The chemist...
Questions

English, 13.05.2021 18:20



Mathematics, 13.05.2021 18:20

Mathematics, 13.05.2021 18:20


Arts, 13.05.2021 18:20

Mathematics, 13.05.2021 18:20




Chemistry, 13.05.2021 18:20

Mathematics, 13.05.2021 18:20

Mathematics, 13.05.2021 18:20


Business, 13.05.2021 18:20

Mathematics, 13.05.2021 18:20

Mathematics, 13.05.2021 18:20

Mathematics, 13.05.2021 18:20

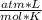 T= 20 C= 293 K (being 0 C= 273 K)
T= 20 C= 293 K (being 0 C= 273 K)