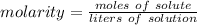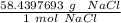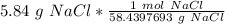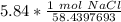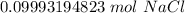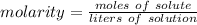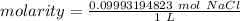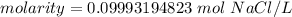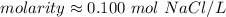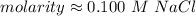Chemistry, 25.07.2021 04:00 brebun4742
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume of 1.00 L. What is the molarity of the solution
Group of answer choices
0.0250 M
0.400 M
0.100 M
1.00 M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume...
Questions




Mathematics, 21.03.2020 11:11


Mathematics, 21.03.2020 11:11






History, 21.03.2020 11:12

Mathematics, 21.03.2020 11:12

Mathematics, 21.03.2020 11:12

Mathematics, 21.03.2020 11:12