
Chemistry, 21.03.2020 11:11 alisonnn101
Calculate ΔS for the isothermal compression of 3.05 mol of Cu(s) from 1.00 bar to 1370. bar at 298 K. α=0.492×10−4K−1,κT=0.78×10−6 bar−1, and the density is 8.92 g⋅cm−3.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2

Chemistry, 23.06.2019 09:30
Organisms that live in the alpine and taiga biomes have developed unique adaptations that aid in their survival. moss campion is one of the plants found in the alpine biome. it has small leaves and a cushion shape that protect it from the wind and freezing temperatures in the alpine. how has the moss campion adapted to enable its survival in the alpine biome? a. waxy needles b. cone-shaped c. thin trunks d. low-growing
Answers: 1

You know the right answer?
Calculate ΔS for the isothermal compression of 3.05 mol of Cu(s) from 1.00 bar to 1370. bar at 298 K...
Questions


Mathematics, 19.10.2021 14:00






Mathematics, 19.10.2021 14:00





Geography, 19.10.2021 14:00

History, 19.10.2021 14:00

Mathematics, 19.10.2021 14:00

Health, 19.10.2021 14:00




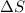 ) is as follows.
) is as follows.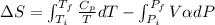
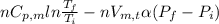
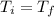
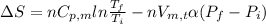
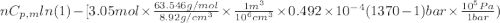 = -0.146 J/K
= -0.146 J/K

