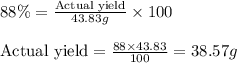Chemistry, 13.07.2021 20:40 chaparro0512
A chemist reacted 17.25 grams of sodium metal with an excess amount of chlorine gas. The chemical reaction that occurred is shown. Na + Cl2 → NaCl If the percentage yield of the reaction is 88%, what is the actual yield? Show your work, including the use of stoichiometric calculations and conversion factors.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
A chemist reacted 17.25 grams of sodium metal with an excess amount of chlorine gas. The chemical re...
Questions


History, 08.12.2020 01:00

Health, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00



English, 08.12.2020 01:00




Biology, 08.12.2020 01:00


English, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Spanish, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00



History, 08.12.2020 01:00

Business, 08.12.2020 01:00

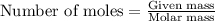 ......(1)
......(1)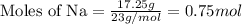
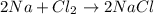
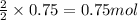 of NaCl
of NaCl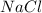 = 58.44 g/mol
= 58.44 g/mol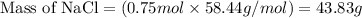
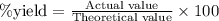 ......(1)
......(1)