
Chemistry, 12.07.2021 01:00 franstirlacci
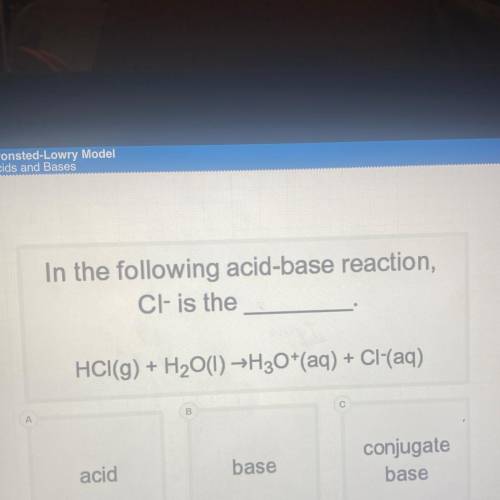
In the following acid-base reaction,
Cl- is the
HCI(g) + H2O(l) →H30+(aq) + Cl(aq)
acid
base
conjugate
base

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
In the following acid-base reaction,
Cl- is the
HCI(g) + H2O(l) →H30+(aq) + Cl(aq)
acid...
HCI(g) + H2O(l) →H30+(aq) + Cl(aq)
acid...
Questions

History, 09.07.2019 15:20

History, 09.07.2019 15:20

Chemistry, 09.07.2019 15:20

Chemistry, 09.07.2019 15:20

History, 09.07.2019 15:20

Mathematics, 09.07.2019 15:20

History, 09.07.2019 15:20

Mathematics, 09.07.2019 15:20

History, 09.07.2019 15:20

Social Studies, 09.07.2019 15:20

Mathematics, 09.07.2019 15:20

Biology, 09.07.2019 15:20


History, 09.07.2019 15:20

Business, 09.07.2019 15:20

Biology, 09.07.2019 15:20

History, 09.07.2019 15:20

Biology, 09.07.2019 15:20

Social Studies, 09.07.2019 15:20



